About VAC4EU
Organisational Structure
Management of VAC4EU is ensured by the following bodies: The General Assembly, the Executive Board and the secretary General and Office. VAC4EU may also create advisory committees: Scientific committees (study specific and open community), Financial Audit and Independent Strategic Advisory Boards and Working Groups within the -Objectives- of VAC4EU in various forms. They are established under the authority of the Executive Board.
Organisational Structure overview
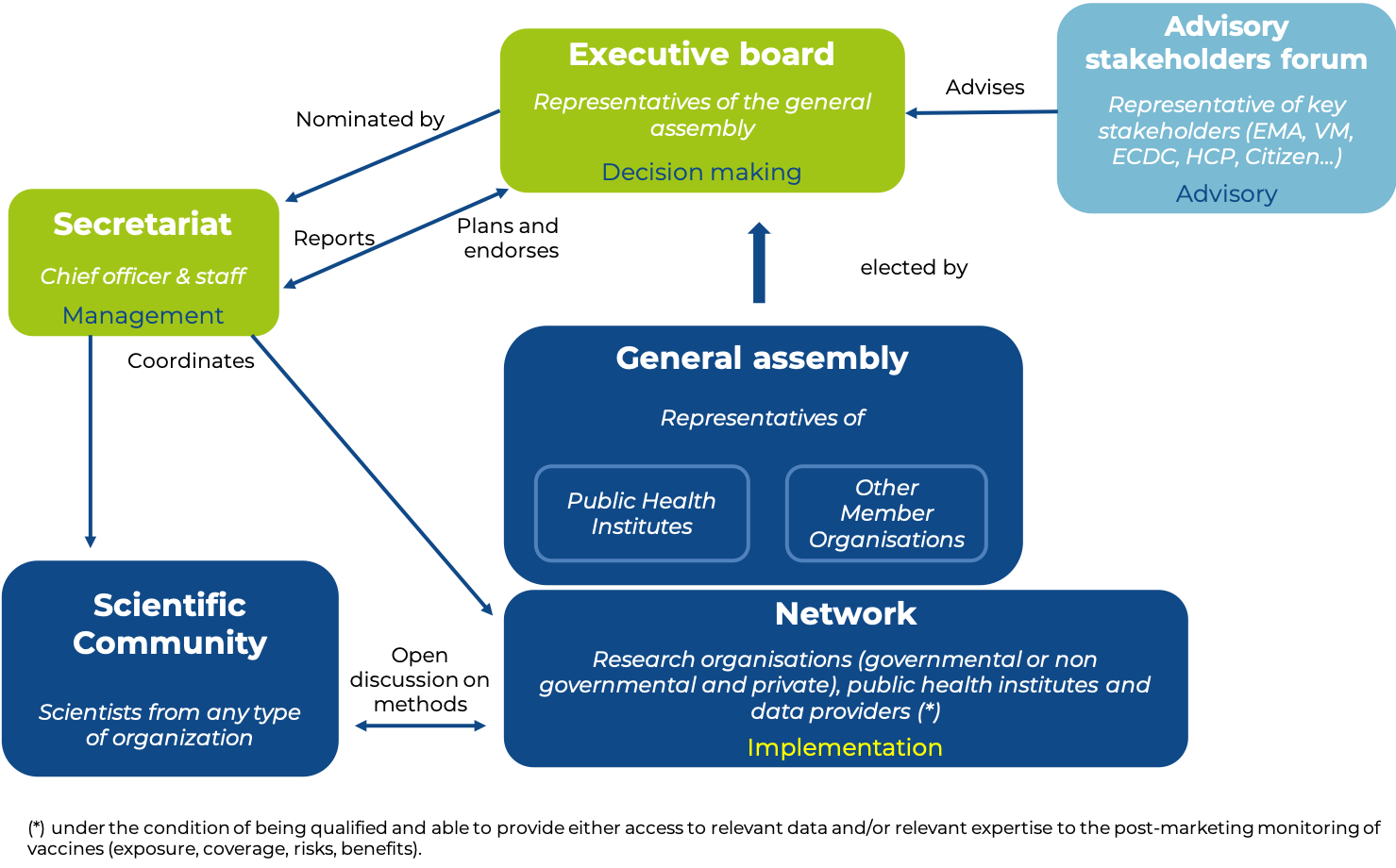
Members of the VAC4EU Association General Assembly can be research organisations (governmental and non-governmental or private), public health institutes, and data access providers under the condition of being qualified and able to provide either access to relevant data and/or relevant expertise to the post-marketing monitoring of vaccines (exposure, coverage, risks, benefits). Publicly listed organizations and drug/vaccine manufacturers cannot be an organizational member of VAC4EU. Applications for Membership shall be addressed in writing to VAC4EU Secretary-General. Membership is granted by the General Assembly upon the proposal of the Executive Board.
The VAC4EU Secretariat is responsible for the following activities
-
Administer and promote VAC4EU
-
Support the work and management of the VAC4EU entities
-
Advocate about the importance of transparent and easy to use evidence on vaccine coverage, benefits and risks
-
Identify and characterize data sources
-
Maintain, update and provide access to the VAC4EU website, data sharing platform, tools and templates
-
Research & business development activities
-
Operate as first contact point and matchmaking for study requests
-
Organize meetings
-
Develop and administer training
The VAC4EU study network is responsible for the following activities
-
Maintain a repository of members and capacity
-
Provide information on data sources (meta-data and database characterization)
-
Conduct vaccine coverage, benefit, risk and benefit/risk studies and monitoring according to VAC4EU endorsed best practice and procedures
-
Provide the community with practical methodological questions
-
Propose, evaluate and implement procedures for generating timely and robust evidence on vaccine coverage, benefits and risks
